Where in the Uterus Does the Baby Implant
Implantation
From Embryology
Jump to:navigation, search
| Embryology - nine Mar 2022 |
|---|
| Google Interpret - select your linguistic communication from the list shown below (this will open a new external folio) |
| العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
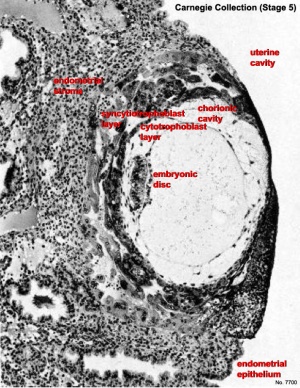
Human conceptus fully implanted (Phase 5).
The term "implantation" is used to depict process of zipper and invasion of the uterus endometrium past the blastocyst (conceptus) in placental animals. In humans, this process begins at the end of week 1, with almost successful human pregnancies the conceptus implants 8 to x days after ovulation, and early pregnancy loss increases with later implantation.[1] The implantation process continues through the 2nd week of development.
The initial phase of the implantation procedure is "adplantation". This first phase requires the newly hatched blastocyst to loosely adhere to the endometrial epithelium, often "rolling" to the eventual site of implantation where it is firmly adhered. This process requires both the blastocyst adhesion interaction with the endometrium during the "receptive window".
Subsequent development of the placenta allows maternal support of embryonic and fetal development. If implantation has non proceeded sufficiently during the menstrual cycle to allow hormonal feedback to the ovary, and then the next cycle may embark leading to conceptus loss. There is also prove, from brute models, that a conceptus with major genetic does not develop or implant correctly leading to their loss during the first and second weeks of development.
In contempo years with the development or Assisted Reproductive Technologies (ART or IVF) in that location is a growing involvement in this process, with techniques that introduce the blastocyst into the uterus to allow normal implantation to occur.
Abnormal implantation is where this process does non occur in the trunk of the uterus (ectopic) or where the placenta forms incorrectly. In improver implantation tin occur ordinarily but with an aberrant conceptus, equally in a hydatiform mole development.
Some Recent Findings
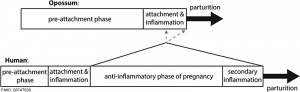
Human and opossum pregnancy[2]
- A hypoxia-induced Rab pathway regulates embryo implantation past controlled trafficking of secretory granules
PNAS "In many mammalian species, embryo implantation and processes of early on pregnancy occur in a hypoxic surroundings. However, the mechanisms underlying maternal adaptation to hypoxia during early on pregnancy remain unclear. This work has uncovered an important mechanism in mammalian reproduction and evolution by identifying maternal secretory granules that mediate molecular dialogue between the maternal tissue compartments during early pregnancy. This dialogue, which is the molecular footing of adaptation to hypoxia, is critical for embryo implantation and institution of pregnancy."
- N-glycosylation of uterine endometrium determines its receptivity [3] "Glycosylation alters the molecular and functional features of glycoproteins, which is closely related with many physiological processes and diseases. During "window of implantation", uterine endometrium transforms into a receptive status to take the embryo, thereby establishing successful embryo implantation. In this commodity, we aimed at investigating the role of N-glycosylation, a major modification type of glycoproteins, in the process of endometrial receptivity establishment. Results found that human uterine endometrial tissues at mid-secretory stage exhibited Lectin PHA-E+50 (recognizes the branched N-glycans) positive Northward-glycans equally measured by the Lectin fluorescent staining assay. By utilizing in vitro implantation model, we found that de-Northward-glycosylation of human endometrial Ishikawa and RL95-two cells past tunicamycin (inhibitor of North-glycosylation) and peptide-N-glycosidase F (PNGase F) impaired their receptive ability to human trophoblastic JAR cells. Meanwhile, Northward-glycosylation of integrin αvβ3 and leukemia inhibitory factor receptor (LIFR) are constitute to play key roles in regulating the ECM-dependent FAK/Paxillin and LIF-induced STAT3 signaling pathways, respectively, thus affecting the receptive potentials of endometrial cells. Furthermore, in vivo experiments and primary mouse endometrial cells-embryos coculture model further verified that N-glycosylation of mouse endometrial cells contributed to the successful implantation. Our results provide new evidence to show that N-glycosylation of uterine endometrium is essential for maintaining the receptive functions, which gives a better understanding of the glycobiology of implantation."
- Dynamics of trophoblast differentiation in peri-implantation-stage human embryos [4] "Single-cell RNA sequencing of cells from cultured man blastocysts has enabled us to define the transcriptomic mural of placental trophoblast (TB) that surrounds the epiblast and associated embryonic tissues during the enigmatic day eight (D8) to D12 peri-implantation period before the villous placenta forms. We analyzed the transcriptomes of 3 early placental cell types, cytoTB (CTB), syncytioTB (STB), and migratoryTB (MTB), picked manually from cultured embryos dissociated with trypsin and were able to follow sublineages that emerged from proliferating CTB at the periphery of the conceptus. A unique course of CTB with some features of STB was detectable at D8, while mature STB was at its zenith at D10. A form of MTB with a mixed MTB/CTB phenotype arose around D10. By D12, STB generation was in pass up, CTB had entered a new phase of proliferation, and mature MTB cells had begun to move from the main body of the conceptus. Notably, the MTB transcriptome at D12 indicated enrichment of transcripts associated with IFN signaling, migration, and invasion and upward-regulation of HLA-C, HLA-E, and HLA-G. The STB, which is distinct from the STB of after villous STB, had a phenotype consequent with intense protein export and placental hormone production, also as migration and invasion. The studies prove that TB associated with human embryos is in rapid developmental flux during peri-implantation period when it must invade, bespeak robustly to the female parent to ensure that the pregnancy continues, and make first contact with the maternal allowed system."
| More contempo papers |
|---|
| This tabular array allows an automated figurer search of the external PubMed database using the listed "Search term" text link.
More than? References | Word Page | Periodical Searches | 2019 References | 2020 References Search term: Human+Embryo Implantation | Embryo Implantation | Ectopic Implantation | Uterine Implantation |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, simply as that listing grew in length take now been shuffled down to this collapsible table. Run into besides the Discussion Page for other references listed by year and References on this current page.
|
Week ane and 2 Man Development Overview
Endometrial Receptivity
In humans, receptivity occurs six days afterwards the post-ovulatory progesterone surge and lasts almost two to 4 days (well-nigh days 20 to 24 of the menstrual bike).
A similar "receptivity window" occurs following fertilization in other species: rat day 5 and mouse day 4.5. Many studies have looked into identifying markers for this receptivity menstruation both to optimise and to block this process.
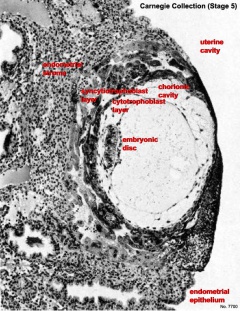
Carnegie Stage five
The conceptus is shown fully implanted in the uterine wall. Uterine wall is shown on the left and the uterine cavity is shown on the right.
| Early Stage 5 | Tardily Stage five |
|---|---|
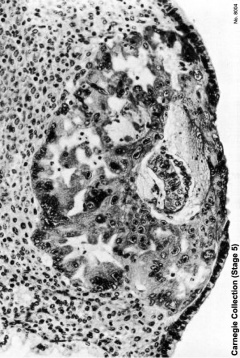 |  |
Implantation Animation
The 2nd calendar week of human development is concerned with the process of implantation and the differentiation of the blastocyst into early embryonic and placental forming structures.
- implantation commences nigh day 6 to seven
- Adplantation - begins with initial adhesion to the uterine epithelium
- blastocyst then slows in motility, "rolls" on surface, aligns with the inner prison cell mass closest to the epithelium and stops
- Implantation - migration of the blastocyst into the uterine epithelium, process complete by about day 9
- coagulation plug - left where the blastocyst has entered the uterine wall day 12
Normal Implantation Sites - in uterine wall superior, posterior, lateral
Uterine Epithelium
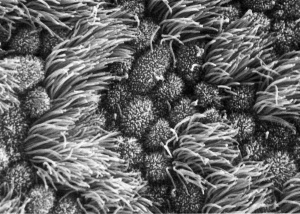
Human uterine tube ciliated epithelium SEM
Uterine epithelial cilia are responsible for the initial motility of the ooycte and conceptus (zygote, morula, blastocyst). In humans, this is during the first week of development. Uterine epithelial microvilli are involved with the implantation procedure. Hormones (estrogen and progesterone) regulate both cilia and microvilli number and structure. The differences in size and shape of cilia and microvilli are shown by scanning micrographs of the lumenal surface of the epithelium lining the mammalian uterine tube.
| Cilia | Microvilli |
|---|---|
|
|
Uterodomes or Pinopods
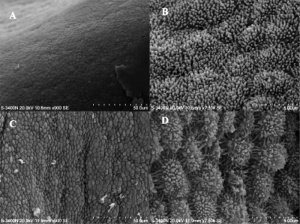
Scanning electron microscope images of the pig endometrial surface of a 24-hour interval thirteen pregnant sow.[11]
(uterodomes) Cellular feature seen on the apical uterine epithelium surface. The presence of these structures is thought in many species to be a marker for endometrial receptivity. In humans though, recent studies have shown pinopodes are also present throughout the luteal stage of the menstrual wheel.[12] It has also been suggested that their part is non primarily pinocytotic, hence the alternate suggested proper name "uterodomes" based upon their appearance when imaged by electron microscopy.[13]
These transient microprotrusions inter-digitate with microvilli on the apical syncytiotrophoblast surface of the blastocyst during initial adplantation and implantation process.
Decidua Secreted Factors
The decidual cells secrete many interleukins, growth factors and other factors that tin can exist classified as either supporting implantation (pro-invasive) or inhibiting (anti-invasive). Note that both processes are required for the normal implantation station process to proceed correctly.
- Pro-invasive factors - IL-1β, IL-5, IL-6, IL-vii, IL-8, IL-nine, IL-13, IL-15, Eotaxin CCL11, IP-10 and RANTES.
- Anti-invasive factors - IL-10, IL-12 and VEGF.
Inhibitory Interactions
The epithelial surface has an associated glycocalyx, the prison cell surface formed by transmembrane and secreted glycoproteins. Mucins are a major components and mucin ane (MUC1), a transmembrane protein appears to have dual roles. Firstly, interim as a barrier to both microbial infection and enzymatic assail. Secondly, its expression is altered past hormones and decreased MUC1 expression associated with receptivity. This suggests that high levels of mucin are inhibitory to implantation. Interestingly the same protein is highly expressed later in the placental amnion, where its anti-bacterial and anti-agglutinative roles are again required.[14]
Adhesive Interactions
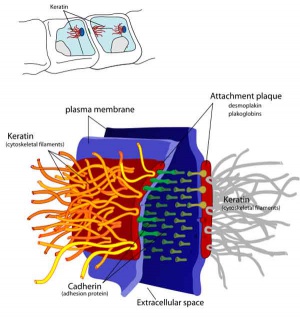
Cascade of endometrial/conceptus adhesive interactions[15]:
- Carbohydrate-mediated binding to the glycocalyx.
- Progressing to tighter binding involving osteopontin (OPN), members of the immunoglobulin superfamily (IgSF), integrin and cadherin families, trophinin and CD44.
- Activation of proteases including MMPs and ADAMs may well be important in these molecular assemblies.
- Lateral epithelial membrane components, including desmosomes, detach and reassemble as trophectoderm extends between maternal epithelial cells.
Implantation Factors
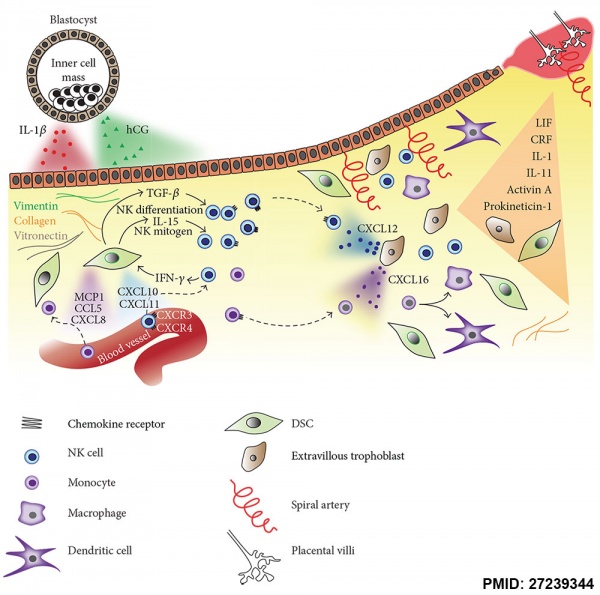
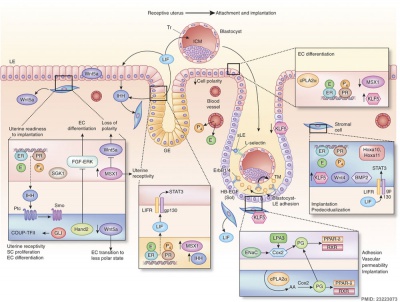
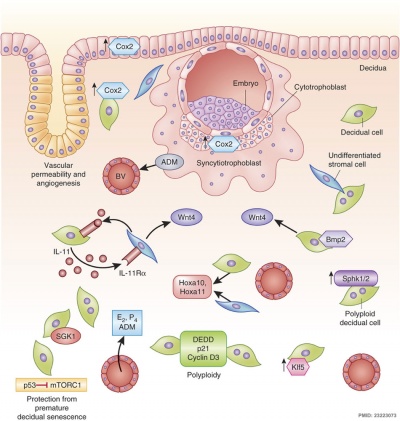
Molecular Implantation and Decidualization[16]
Trophinin
- Trophinin is a membrane protein expressed on blastocyst trophectoderm cells and on uterine endometrium epithelial cells.
- Adhesion is thought to occur through trophinin-trophinin binding.
- Adhesion also triggers ii trophinin mediated effects:
- trophectoderm cells activate for implantation (proliferation, invasion)
- maternal endometrial epithelial cells induced programmed cell death (apoptosis).[17]
- Links: OMIM
Mucin 1
Mucin 1 is a transmembrane protein expressed on the apical borders of secretory epithelial cells including the placental amnion [xiv] and mammary cells.[18]
- Links: OMIM - Mucin ane
Cytokines
In mice, endometrial secretion of 2 IL-6 family cytokines, leukemia inhibitory factor (LIF) and Interleukin-11 (IL-11), are key requirements for implantation. A recent man written report suggests that there is a similar requirement for human conceptus implantation.[19]
Uterine Leukemia Inhibitory Factor (LIF) Expression[twenty]

| a - At day 4 of pregnancy, oestrogen E2 induces LIF expression in the endometrial glands, leading to LIF secretion into the uterine lumen. There, LIF binds to its receptors on the surface of epithelial cells. | b - This makes the uterus receptive to the blastocyst, which implants by day 5 of pregnancy. Hu et al. discover that LIF expression in the endometrial glands also depends on the regulatory action of p53. In the absence of p53, insufficient LIF is produced, the uterus does not become adequately receptive, and fewer blastocysts implant. |
Galectin 9
Galectin 9, a poly peptide that binds galactosides and has many different roles, has been identified as a marker for the mid- and tardily-secretory phases of human endometrium and decidua. The loftier expression at uterodomes during the flow of implantation, suggests that it may as well mark endometrial receptivity.[21]
- Links: OMIM - leukemia inhibitory gene | OMIM - Interleukin-eleven | OMIM - Galectin 9
Day 8 to 9
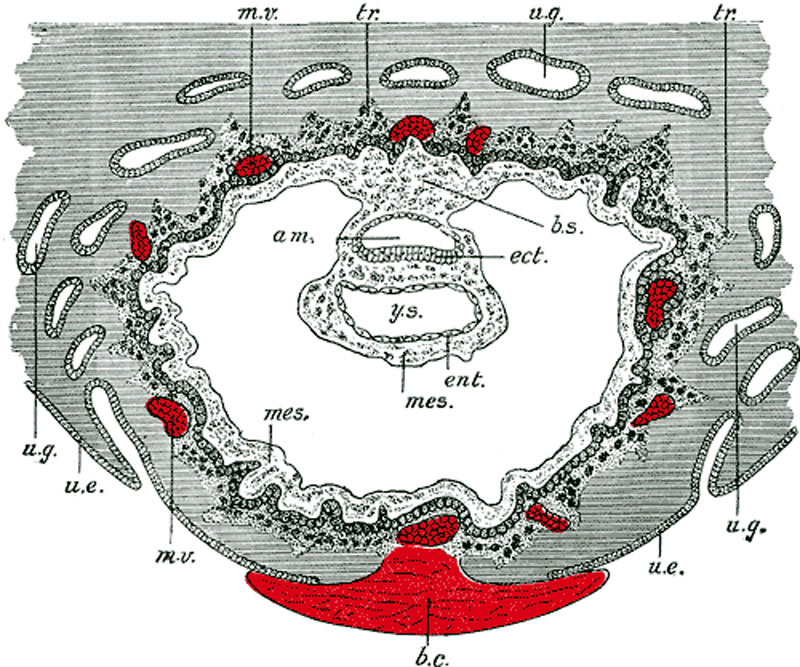
- am. - amniotic cavity
- b.c. - claret clot, at the site of initial implantation
- b.s. - body-stalk, or connective stalk later on forming the placental string region with placental blood vessels
- ect. - embryonic ectoderm that volition contribute to embryonic and placental membrane development
- ent. - entoderm (endoderm), this was the celebrated term for what we today call endoderm that will contribute to embryo evolution
- mes. - mesoderm, consisting of both embryonic mesoderm (in the trilaminar embryonic disc) and extraembryonic mesoderm (exterior the trilaminar embryonic disc)
- m.v. - maternal vessels, spiral arteries that take been opened at their ends
- tr. - trophoblast, relative to the embryonic disc the outer syncitiotrophoblast and inner cytotrophoblast layers that volition contribute to placental evolution
- u.e. - uterine epithelium, the epithelial layer that lines the unerus
- u.g. - uterine glands, the glands that secrete nutrients to back up the initial growth both before and subsequently implantation
- y.s. - yolk-sac, the endoderm lined and extraembryonic mesoderm covered cavity that will contribute to the alimentary canal, blood and blood vessels
Maternal Immune
How does the implanting conceptus avoid immune rejection by the maternal allowed system? At that place are a number of maternal and embryonic mechanisms that are thought to act to prevent immune rejection of the implanting conceptus.
Maternal Allowed
How does the implanting conceptus avoid immune rejection by the maternal immune system? There are a number of maternal and embryonic mechanisms that are idea to act to forbid immune rejection of the implanting conceptus, though the complete mechanism(s) are unknown. This is particularly relevant to Assisted Reproductive Technologies involving donor eggs.
Below are some examples of research on this topic.
| Decidual Allowed Cells | ||
|---|---|---|
| Decidual Macrophages (Mϕ) | Decidual T cells | Uterine Natural Killer cells |
|
|
|
Chemokine Cistron Silencing
- Remove the attraction of maternal immune cells.
A mouse study[6] has shown that the normal immune response to inflammation, accumulation of effector T cells in response to chemokine secretion does non occur during implantation. This is prevented locally by epigenetic silencing of chemokine expression in the decidual stromal cells.
Corticotropin-Releasing Hormone
- Kill the maternal immune cells.
Both maternal and implanting conceptus release CRH at the embryo implantation site. This hormone then binds to receptors on the surface of trophoblast (extravillous trophoblast) cells leading to expression of a poly peptide (Fas ligand, FasL) that activates the extrinsic cell expiry pathway on any local maternal immune cells ( T and B lymphocytes, natural killer cells, monocytes and macrophages).[22] This cannot be the only machinery, equally mice with dysfunctional FasL proteins are still fertile.
- Links: Immune System Evolution
Decidual Reaction
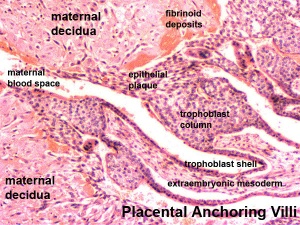
During pregnancy, at implantation the endometrium is altered by the maternal steroid hormones, estrogen and progesterone and in response to the implanting conceptus and renamed the "decidua". This process of signaling is called the decidual reaction or decidualization, and commences at the site of implantation and spreads throughout the uterine lining.
- occurs inside the uterus wall
- initially at site of implantation and includes both cellular and matrix changes
- reaction spreads throughout entire uterus, not at cervix
- promoted by the maternal steroid hormones, estrogen and progesterone
- extensive proliferation and differentiation of uterine stromal cells
- degradation of fibrinoid and glycogen and epithelial plaque formation (at anchoring villi)
- presence of decidual cells are indicative of pregnancy
In uterine stromal decidualization, os morphogenetic protein ii (BMP2) nonactive precursor protein is cleaved by proprotein convertase v/6 (PC6) to produce the active course. Deletion or knockdown of either BMP2 or PC6 inhibits decidualization leads to implantation failure and female person infertility.[23]
A recent human histological report has shown that endometrial stromal jail cell decidualization leads to a loss of lymphatics particularly credible around the uterine spiral arteries.[24]
- Links: OMIM - bone morphogenetic protein ii | OMIM - proprotein convertase 5
Cervical Mucus Plug
Along with the decidualization, estrogen also stimulates the production of fungus from glands at the opening of the uterus, the cervix, where it joins the vagina. This secreted fungus then forms a plug/barrier (CMP) acting in a mechanical and antibacterial manner.
Aberrant Implantation
Tubal Pregnancy
Abnormal implantation sites or Ectopic Pregnancy occurs if implantation is in uterine tube or outside the uterus.
- sites - external surface of uterus, ovary, bowel, gastrointestinal tract, mesentry, peritoneal wall
- If non spontaneous then, embryo has to exist removed surgically
Tubal pregnancy - 94% of ectopic pregnancies
- if uterine epithelium is damaged (scarring, pelvic inflammatory illness)
- if zona pellucida is lost too early, allows premature tubal implantation
- embryo may develop through early stages, can erode through the uterine horn and reattach within the peritoneal cavity
- Links: Ectopic Pregnancy | Movie - Ectopic pregnancy ultrasound
Hydatidiform Mole
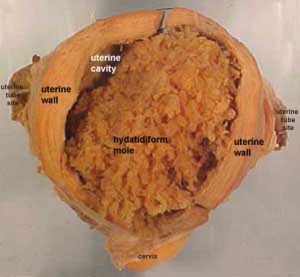
Another type of abnormality is when just the conceptus trophoblast layers proliferates and not the embryoblast, no embryo develops, this is called a "hydatidiform mole", which is due to the continuing presence of the trophoblastic layer, this abnormal conceptus can as well implant in the uterus. The trophoblast cells will secrete human chorionic gonadotropin (hCG), as in a normal pregnancy, and may appear maternally and by pregnancy test to be "normal". Prenatal diagnosis past ultrasound analysis demonstrates the absence of a embryo.
There are several forms of hydatidiform mole: fractional mole, complete mole and persistent gestational trophoblastic tumor. Many of these tumours arise from a haploid sperm fertilizing an egg without a female pronucleus (the alternative grade, an embryo without sperm contribution, is called parthenogenesis). The tumour has a "grape-similar" placental appearance without enclosed embryo formation. Following a beginning molar pregnancy, there is approximately a 1% risk of a 2nd tooth pregnancy.
This topic is besides covered in Placenta - Abnormalities
Abnormal Placentation
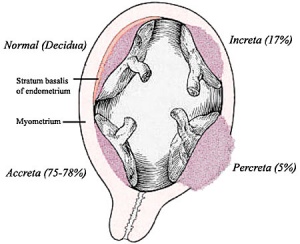
Placental implantation abnormalities
Abnormalities tin can range from anatomical associated with degree or site of inplantation, structure (as with twinning), to placental part, placento-maternal effects (pre-eclampsia, fetal erythroblastosis) and finally mechanical abnormalities associated with the placental (umbilical) cord.
This topic is also covered in Placenta - Abnormalities
References
- ↑ Wilcox AJ, Baird DD & Weinberg CR. (1999). Time of implantation of the conceptus and loss of pregnancy. Due north. Engl. J. Med. , 340, 1796-9. PMID: 10362823 DOI.
- ↑ ii.0 2.ane Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R & Wagner GP. (2017). Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. U.South.A. , 114, E6566-E6575. PMID: 28747528 DOI.
- ↑ Yu M, Qin H, Wang H, Liu J, Liu Southward & Yan Q. (2020). N-glycosylation of uterine endometrium determines its receptivity. J. Cell. Physiol. , 235, 1076-1089. PMID: 31276203 DOI.
- ↑ West RC, Ming H, Logsdon DM, Sun J, Rajput SK, Kile RA, Schoolcraft WB, Roberts RM, Krisher RL, Jiang Z & Yuan Y. (2019). Dynamics of trophoblast differentiation in peri-implantation-stage human embryos. Proc. Natl. Acad. Sci. U.Southward.A. , 116, 22635-22644. PMID: 31636193 DOI.
- ↑ Santillán I, Lozano I, Illán J, Verdú 5, Coca Due south, Bajo-Arenas JM & Martinez F. (2015). Where and when should natural killer cells exist tested in women with repeated implantation failure?. J. Reprod. Immunol. , 108, 142-8. PMID: 25708533 DOI.
- ↑ 6.0 half-dozen.1 Nancy P, Tagliani E, Tay CS, Asp P, Levy DE & Erlebacher A. (2012). Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science , 336, 1317-21. PMID: 22679098 DOI.
- ↑ Domínguez F, Simón C, Quiñonero A, Ramírez MÁ, González-Muñoz E, Burghardt H, Cervero A, Martínez South, Pellicer A, Palacín Yard, Sánchez-Madrid F & Yáñez-Mó K. (2010). Homo endometrial CD98 is essential for blastocyst adhesion. PLoS ONE , 5, e13380. PMID: 20976164 DOI.
- ↑ Cakmak H & Taylor HS. (2011). Implantation failure: molecular mechanisms and clinical treatment. Hum. Reprod. Update , 17, 242-53. PMID: 20729534 DOI.
- ↑ Duzyj CM, Barnea ER, Li G, Huang SJ, Krikun G & Paidas MJ. (2010). Preimplantation cistron promotes first trimester trophoblast invasion. Am. J. Obstet. Gynecol. , 203, 402.e1-4. PMID: 20708167 DOI.
- ↑ Fang LQ, Zhang H, Ding XY, Li DQ, Hou XL, Qiao H, Bai J & Wang ZB. (2010). Mouse trophoblastic cells exhibit a ascendant invasiveness phenotype over cancer cells. Cancer Lett. , 299, 111-eight. PMID: 20826050 DOI.
- ↑ Ren Q, Guan South, Fu J & Wang A. (2010). Temporal and spatial expression of Muc1 during implantation in sows. Int J Mol Sci , 11, 2322-35. PMID: 20640155 DOI.
- ↑ Quinn CE & Casper RF. (2009). Pinopodes: a questionable office in endometrial receptivity. Hum. Reprod. Update , 15, 229-36. PMID: 18997181 DOI.
- ↑ Murphy CR. (2000). Agreement the apical surface markers of uterine receptivity: pinopods-or uterodomes?. Hum. Reprod. , fifteen, 2451-4. PMID: 11098008
- ↑ 14.0 fourteen.one Sood R, Zehnder JL, Druzin ML & Brown PO. (2006). Cistron expression patterns in man placenta. Proc. Natl. Acad. Sci. U.S.A. , 103, 5478-83. PMID: 16567644 DOI.
- ↑ Singh H & Aplin JD. (2009). Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. J. Anat. , 215, 3-13. PMID: 19453302 DOI.
- ↑ Cha J, Sun X & Dey SK. (2012). Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. , 18, 1754-67. PMID: 23223073 DOI.
- ↑ Tamura N, Sugihara K, Akama TO & Fukuda MN. (2011). Trophinin-mediated cell adhesion induces apoptosis of human endometrial epithelial cells through PKC-δ. Cell Cycle , 10, 135-43. PMID: 21191175 DOI.
- ↑ Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat Northward, Burchell J, Pemberton L, Lalani EN & Wilson D. (1990). Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. , 265, 15286-93. PMID: 1697589
- ↑ Marwood M, Visser Yard, Salamonsen LA & Dimitriadis E. (2009). Interleukin-xi and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology , 150, 2915-23. PMID: 19213836 DOI.
- ↑ Hu W, Feng Z, Teresky AK & Levine AJ. (2007). p53 regulates maternal reproduction through LIF. Nature , 450, 721-4. PMID: 18046411 DOI.
- ↑ Shimizu Y, Kabir-Salmani 1000, Azadbakht M, Sugihara K, Sakai Thou & Iwashita Yard. (2008). Expression and localization of galectin-ix in the human uterodome. Endocr. J. , 55, 879-87. PMID: 18506087
- ↑ Makrigiannakis A, Zoumakis Eastward, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravanis A & Chrousos GP. (2001). Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat. Immunol. , 2, 1018-24. PMID: 11590404 DOI.
- ↑ Heng S, Paule S, Hardman B, Li Y, Singh H, Rainczuk A, Stephens AN & Nie Thou. (2010). Posttranslational activation of bone morphogenetic poly peptide 2 is mediated past proprotein convertase 6 during decidualization for pregnancy establishment. Endocrinology , 151, 3909-17. PMID: 20555025 DOI.
- ↑ Volchek M, Girling JE, Lash GE, Cann L, Kumar B, Robson SC, Bulmer JN & Rogers PA. (2010). Lymphatics in the human endometrium disappear during decidualization. Hum. Reprod. , 25, 2455-64. PMID: 20729537 DOI.
Reviews
Play a trick on C, Morin S, Jeong JW, Scott RT & Lessey BA. (2016). Local and systemic factors and implantation: what is the evidence?. Fertil. Steril. , 105, 873-84. PMID: 26945096 DOI.
Cha J, Lord's day 10 & Dey SK. (2012). Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. , 18, 1754-67. PMID: 23223073 DOI.
Hannan NJ, Evans J & Salamonsen LA. (2011). Alternating roles for allowed regulators: establishing endometrial receptivity for implantation. Expert Rev Clin Immunol , seven, 789-802. PMID: 22014020 DOI.
Paulson RJ. (2011). Hormonal induction of endometrial receptivity. Fertil. Steril. , 96, 530-5. PMID: 21880274 DOI.
Fukuda MN & Sugihara K. (2008). An integrated view of Fifty-selectin and trophinin function in human embryo implantation. J. Obstet. Gynaecol. Res. , 34, 129-36. PMID: 18412772 DOI.
Aplin JD. (2006). Embryo implantation: the molecular mechanism remains elusive. Reprod. Biomed. Online , xiii, 833-nine. PMID: 17169205
Articles
Fukuda MN & Sugihara K. (2007). Signal transduction in human embryo implantation. Cell Cycle , half dozen, 1153-vi. PMID: 17495530 DOI.
Search PubMed
Search Pubmed: Embryo Adplantation | Embryo Implantation | tubal pregnancy | Endometrial Receptivity | Placenta Abnormalities | Pinopods | decidualization
Embryo Week: Week ane | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Calendar week 7 | Week eight | Calendar week 9
- Carnegie Stages: i | 2 | three | four | 5 | half dozen | 7 | eight | nine | x | 11 | 12 | 13 | 14 | xv | 16 | 17 | 18 | 19 | twenty | 21 | 22 | 23 | Nearly Stages | Timeline
| Carnegie Drove - Stage v | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serial No. | Stage | Course | Fixative | Embedding Medium | Thinness (µm) | Stain | Year | Notes | ||
| 8020 | 5a | Exc. | Alc. & Bouin | C-P | 6 | (Stain - Haematoxylin Eosin) | 1942 | Hertig and Rock (1945a)[ane] | ||
| 8155 | 5a | Exc. | Bouin | C-P | vi | (Stain - Haematoxylin Eosin) | 1943 | Hertig and Stone (1949)[2] | ||
| 8225 | 5a | Exc. | Alc. & Bouin | C-P | 6 | (Stain - Haematoxylin Eosin) | 1944 | Hertig and Rock (1945b)[3] | ||
| 8004 | 5b | Exc | Alc. & Bouin | C-P | 6 | (Stain - Haematoxylin Eosin) | 1942 | Hertig and Rock (1945a)[1] | ||
| 8171 | 5b | Exc | Alc. | C-P | six | (Stain - Haematoxylin Eosin) | 1943 | Hertig and Rock (1949)[2] | ||
| 8215 | 5b | Exc | Alc. & Bouin | C-P | six | (Stain - Haematoxylin Eosin) | 1944 | Hertig and Rock (1945c)[4] | ||
| 9350 | 5b | Exc | Bouin | ? | ? | (Stain - Haematoxylin Eosin) | 1955 | Heuser (1956)[five] | ||
| 4900 | 5c | Poor | p | P | x | p | 1925 | Incomplete. Streeter (1926)[6] | ||
| 7699 | 5c | Exc. | Bouin | C-P | six | (Stain - Haematoxylin Eosin) | 1939 | Hertig and Rock (1941)[7] | ||
| 7700 | 5c | Exc. | Bouin | C-P | vi | (Stain - Haematoxylin Eosin) | 1938 | Hertig and Rock (1941)[7] | ||
| 7771 | 5c | Exc. | Bouin | C-P | 10 | (Stain - Haematoxylin Eosin) | 1940 | Abnormal | ||
| 7950 | 5c | Exc. | Alc. & Bouin | C-P | 6 | (Stain - Haematoxylin Eosin) | 1941 | Hertig and Rock (1944)[8] | ||
| 8000 | 5c | Poor | Alc. & Bouin | C-P | 8 | (Stain - Haematoxylin Eosin) | 1942 | Abnormal | ||
| 8139 | 5c | Exc. | ? | C-P | half dozen | (Stain - Haematoxylin Eosin) | 1943 | Incomplete. Marchetti (1945)[9] | ||
| 8299 | 5c | Exc. | Alc. & Bouin | C-P | 6 | (Stain - Haematoxylin Eosin), phlox. | 1945 | Abnormal | ||
| 8329 | 5c | Exc. | Alc. & Bouin | C-P | 6 | (Stain - Haematoxylin Eosin), phlox. | 1945 | Abnormal | ||
| 8330 | 5c | Exc. | Alc. & Bouin | C-P | half dozen | (Stain - Haematoxylin Eosin), phlox. | 1945 | |||
| 8370 | 5c | Poor | Alc. & Bouin | C-P | 6 | (Stain - Haematoxylin Eosin), phlox. | 1946 | Abnormal | ||
| 8558 | 5c | Exc. | Alc. & Bouin | C-P | vi | (Stain - Haematoxylin Eosin) | 1947 | |||
| Stage v was originally subdivided into 3 sequential parts a, b, c. Abbreviations
| ||||||||||
References
| ||||||||||
Glossary Links
- Glossary: A | B | C | D | E | F | Thousand | H | I | J | K | Fifty | M | North | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this folio: Hill, M.A. (2022, March ix) Embryology Implantation. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Implantation
-
- What Links Here?
- © Dr Mark Hill 2022, UNSW Embryology ISBN: 978 0 7334 2609 four - UNSW CRICOS Provider Code No. 00098G
Source: https://embryology.med.unsw.edu.au/embryology/index.php/Implantation

0 Response to "Where in the Uterus Does the Baby Implant"
Post a Comment